Antibiotic resistance in animals is a growing issue that urgently needs to be addressed. Antimicrobial agents are frequently used in animal husbandry as an alternative or supplement to feed additives such as antibiotics, and this prophylactic scheme/model/pattern has contributed to the development of antimicrobial resistance. The overuse of antimicrobials can lead to resistant strains developing; for example, when antibiotics are routinely given for minor ailments which would otherwise heal on their own. This post will explore several possible solutions and explain how we can avoid a future where our only option is to rearrange/remodel the established treatment methods for humans, which no longer provide desired effects.
The problem of antibiotic-resistant bacteria

The moment of introducing antibiotics was a turning point in medicine. Antibiotics revolutionized the industry, displacing other methods of combating microorganisms, and contributed to the development of various medical therapies, e.g., chemotherapy, and transplantation. It appeared to be a bold, new world and everyone was on board. People put their trust in this medical marvel. The phenomenon of antibiotic resistance appeared at the beginning of antibiotic discovery, but due to the rate of discovery and introduction of new antibiotics, it did not raise much concern. However, with time, the amount of new and effective antibiotics has drastically decreased, and the problem of antibiotic resistance has become more noticeable. Even before commercially available antibiotics were introduced, this had always been a cause of concern as a natural process. While natural compounds functioned as antibacterials in low doses, therapeutic doses accelerated the healing, as well as the resistance rate. Like all living organisms, Bacteria want to survive. They develop mechanisms that adapt them to unfavorable conditions, e.g. make them resistant to therapeutic agents. Unfortunately, antibiotic resistance is significantly influenced by their overuse, not only in the treatment of diseases, but also when used as growth promoting agents in plant cultivation and animal breeding. Regarding the latter, up to 73% of antibiotics are used worldwide. The greatest consumption is in aquaculture, where even tons of antibiotics are released into the waters and consequently accumulate in the environment. This affects not only the development of antibiotic resistance but also the destruction of the ecosystem. Resolving the antibiotic resistance in animals became an issue that must be addressed right away.
How does Bacteria develop resistance?
Bacteria acquire resistance through gene mutations and horizontal gene transfer: conjugation (collection/transfer of genetic material from/to another bacteria), transformation (collection of genetic material from the environment), transduction (with the participation of bacteriophages). Moreover, bacterial cells can achieve transient, genetically uncoded resistance through processes such as growth in biofilms, swarming adaptation, metabolic dormancy, and persistence.
Looking for other strategies is desirable
Due to the declining effectiveness of antibiotics, it is important to slow down the process of acquiring antibiotic resistance by bacteria and to seek and develop other methods of disease prevention and treatment. Introduction of prescription-only antibiotics would help greatly, as in many countries the access is too easy. Discouraging the antibiotics’ abuse will help slow down the process of developing resistance.
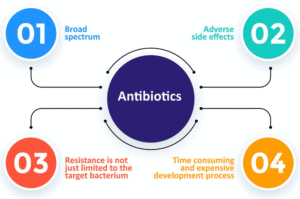 The use of antibiotics in feed and aquaculture must also be eliminated to the necessary minimum. This problem has been discussed many times in the European Union and it was assumed that in 2022 there will be introduced a law banning the use of antibiotics which are of particular importance in medicine. Even for animals, antibiotics should be available by prescription-only, after dispensation by a veterinarian.
The use of antibiotics in feed and aquaculture must also be eliminated to the necessary minimum. This problem has been discussed many times in the European Union and it was assumed that in 2022 there will be introduced a law banning the use of antibiotics which are of particular importance in medicine. Even for animals, antibiotics should be available by prescription-only, after dispensation by a veterinarian.
Increasing the availability of rapid diagnostic tests to detect the etiology of the disease is essential. The use of antibiotics to combat viral infections must be strongly discouraged. This includes cases of pharyngitis, the viral case for which doctors often recommend antibiotic treatments.
Additionally, vaccines are an important preventive measure to help develop immunity against various diseases.
Despite all of the above, the most essential way to bypass this problem of resistance is the use of bacteriophages.
Finding new ways to resolve the issue of developing antibiotic resistance for animals in future is important.
Phage therapy as a “rediscovered” path
Bacteriophages are bacterial viruses that recognize specific species and even strains of bacteria. The name also means “bacterium eater”. Phage therapy was introduced a century ago but was discontinued after the introduction of antibiotics. However, research was continued in places such as Russia, Georgia, and Poland. Institutions which are well-known for their long-term activity in the field of bacteriophages are Eliava Institute of Bacteriophage, Microbiology and Virology, founded in 1923 in Tbilisi, Georgia and the Ludwik Hirszfeld Institute of Immunology and Experimental Therapy, founded in 1952 in Wroclaw, Poland.
 Initially, bacteriophages were used mainly in the treatment of typhoid fever, dysentery, skin and surgical wound infections, peritonitis, septicemia, urinary tract infections, and external otitis, but also in pneumonia, meningitis, osteomyelitis, and postsurgical infections in cancer patients. The allegations that questioned the effectiveness of the bacteriophages mainly included insufficient methodology design. Bacteriophages have regained the interest through a more detailed understanding of phage biology, genetics, immunology, and pharmacology. Many studies suggest that properly developed bacteriophage cocktails give very satisfactory results, both in medicine, agriculture, and aquaculture.
Initially, bacteriophages were used mainly in the treatment of typhoid fever, dysentery, skin and surgical wound infections, peritonitis, septicemia, urinary tract infections, and external otitis, but also in pneumonia, meningitis, osteomyelitis, and postsurgical infections in cancer patients. The allegations that questioned the effectiveness of the bacteriophages mainly included insufficient methodology design. Bacteriophages have regained the interest through a more detailed understanding of phage biology, genetics, immunology, and pharmacology. Many studies suggest that properly developed bacteriophage cocktails give very satisfactory results, both in medicine, agriculture, and aquaculture.
Phage therapy has a good chance of success
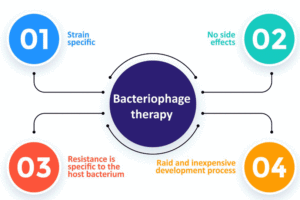
The use of bacteriophages is promising because they act very specifically. This means that they will not act negatively on the microflora, unlike antibiotics. The undoubted advantages are inactivation after they neutralize all target bacteria and spontaneous reproduction. Therefore, small, and single doses are usually sufficient.
Moreover, bacteria striving to develop resistance against phages have a much more difficult task, because phages counteract and through evolution bypass the bacterial defense mechanisms. The tendency of bacteria to develop phage resistance is about ten times slower than in case of antibiotics. Also, phage-resistant bacteria do not possess such qualities in regards to other phages, which still have similar target range. Lastly, phage-resistancy should not be always associated with negative effects. It frequently effects in a decline in bacterial virulence because of the lost ability for colonization and an increase in sensitivity to other phages.
The bacteriophages mode of action may seem/(appear as) to be a disadvantage for some people, and in fact it is a limitation, but mainly in the context of sterilizing, e.g., laboratory equipment and surfaces or limiting the number of microorganisms in food products. However, for the treatment of diseases, specificity is highly desirable. Animals, including humans, are colonized by numerous bacteria that enable the proper functioning of the body, and killing them can lead to troublesome and long-term consequences, such as chronic diarrhea and susceptibility to various diseases. Living organisms come into daily contact with pathogenic bacteria, which are eliminated by the immune system. Microbiota also participates in this process, since it competes for a place to live, preventing other bacteria from growing in their vicinity. Moreover, the problem with narrow spectrum of action can be easily circumvented by creating cocktails containing different phages.
Another reason in favor of phage therapy is the high prevalence of viruses, thanks to which they are easily found in the environment. Moreover, there are many naturally occurring virulent bacteriophages which are lethal to bacteria, and additionally they constantly evolve parallel to the bacteria. Many antibiotics only inhibit multiplication of bacteria instead of killing them, which increases their ability to adapt.
Currently, large technological advances, including the development of efficient tools and techniques, make it possible to significantly increase the effectiveness of phage therapies. Thanks to novel bioinformatics tools and sequencing technology, it is very easy and quick to determine whether the tested bacteriophage is virulent or only inhibits the growth of bacteria. It is also possible to identify the best conditions for a given bacteriophage in which it is most active. As a result, various stabilizing solutions are created that maintain effectiveness and bioactivity even for months.
Disadvantageous factors which must be paid special attention/that should be highlighted are thermal and pH stability. We must bear in mind that Many bacteriophages are most active at neutral pH. The creation of bacteriophage cocktails offers great opportunities to target potentially pathogenic bacteria, while preserving the microflora. Moreover, the constantly expanding knowledge and technical possibilities will contribute to the development of increasingly effective production methods on an industrial scale, and to the reduction of production costs, which are already relatively cheap.
In every sense, bacteriophages are the present and the future. The revolution has begun and will be the new marvel that medicine desperately needs.
Prepared By: Justyna Andrysiak
Gordillo Altamirano FL, Barr JJ. Phage therapy in the post antibiotic era. Clin Microbiol Rev. 2019;32(2):e00066–18. Chan BK, Abedon ST, Loc-Carrillo C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013;8(6):769–783. Loc-Carrillo C, Abedon ST. Pros and cons of phage therapy. Bacteriophage. 2011;1(2):111–114. Reardon S. Phage therapy gets revitalized. Nature. 2014;510(7503):15–16. Kowalska JD, Kazimierczak J, Sowińska PM, Wójcik EA, Siwicki AK, Dastych J. Growing trend of fighting infections in aquaculture environment-opportunities and challenges of phage therapy. Antibiotics (Basel). 2020;9(6):301.