Standardized Botanical Powders (SBPs): Part 5 of 5
 In the last decade, the worldwide organic food market has developed at an exponential rate. Consumers’ attention has shifted from cost-effective products to quality-effective products. This has sparked an organic revolution, which, according to market forecasts, is here to stay. From $15.01 billion in 2021, the worldwide organic meat products market is predicted to reach $20.83 billion in 2025. This demonstrates that a natural lifestyle, and thus nature-based products, are the market’s future. The poultry business must hunt for non-synthetic dietary supplements to improve the health quotient of their flocks to satisfy this high-quality, efficient product demand. With this in mind, we created a series of articles on standardized botanical powders (SBPs), outlining the importance of SBPs, the advantages of using SBPs, the factors that affect their manufacture, and factors to consider when procuring raw material for their manufacture, among other things.
In the last decade, the worldwide organic food market has developed at an exponential rate. Consumers’ attention has shifted from cost-effective products to quality-effective products. This has sparked an organic revolution, which, according to market forecasts, is here to stay. From $15.01 billion in 2021, the worldwide organic meat products market is predicted to reach $20.83 billion in 2025. This demonstrates that a natural lifestyle, and thus nature-based products, are the market’s future. The poultry business must hunt for non-synthetic dietary supplements to improve the health quotient of their flocks to satisfy this high-quality, efficient product demand. With this in mind, we created a series of articles on standardized botanical powders (SBPs), outlining the importance of SBPs, the advantages of using SBPs, the factors that affect their manufacture, and factors to consider when procuring raw material for their manufacture, among other things.
Natural Remedies is the number 1 veterinary herbal healthcare company in India with presence in more than 30 countries across the globe. Through its world-class Research and Development centre, Natural Remedies offers a category of science-based Phytogenic feed additives, called Standardised Botanical Powders (SBPs). In this series of articles, Dr. Raina Raj, Head of Marketing at Natural Remedies, provides in-depth knowledge of what SBPs are, and their benefits in the poultry diet.
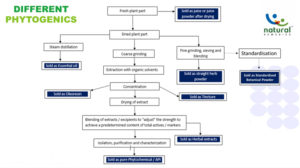
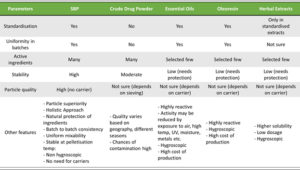
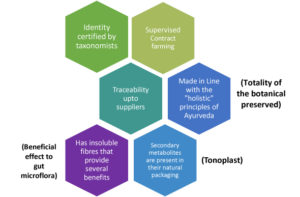
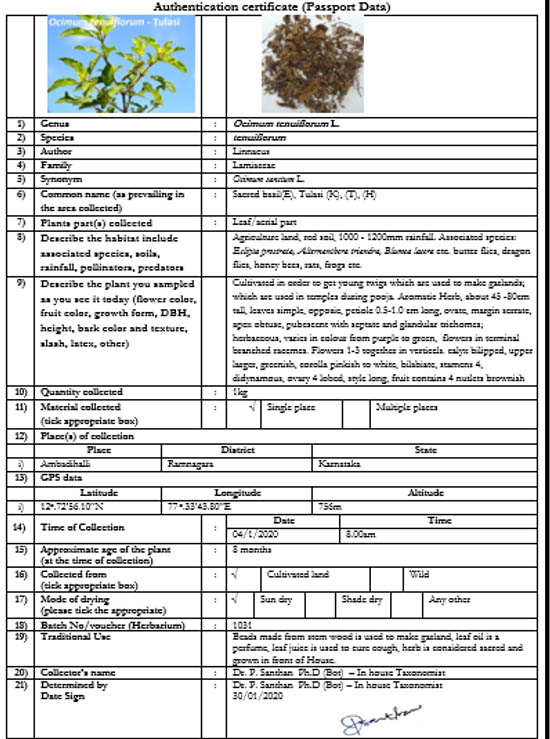
A need for standardization of herbal products exists due to the inherent variation of endogenous phytochemicals found in plants. Farming practices, plant age, soil conditions, geographical location, weather conditions, harvest time, post-harvest processing, and a range of other factors can all affect the phytochemical signature of a given species of plant, resulting in uneven final herbal product outcomes. Standardization ensures the herbal powders’ consistent quality and, as a result, their biological health effects.

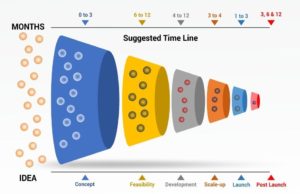
The process of standardizing is not limited to the manufacturing plant but starts at the farm where the herbs are grown. One of the strategies we outlined in this series for procuring superior quality raw botanicals with the least variability in their phytoconstituents is through contracted supervised farming. We have also detailed the process of how an SBP comes into being through aggressive scrutinization through the stage-gate innovation process. The stage-gate innovation process can be segmented as the ideation stage, concept stage, feasibility stage, developmental stage, scale-up stage, launch stage, and post-marketing surveillance. The SBPs are evaluated for their biological efficacy throughout the stage-gate process. In the current issue, we shall highlight the importance of studying the biological impact of the SBPs from the performance of the birds to genomic level.
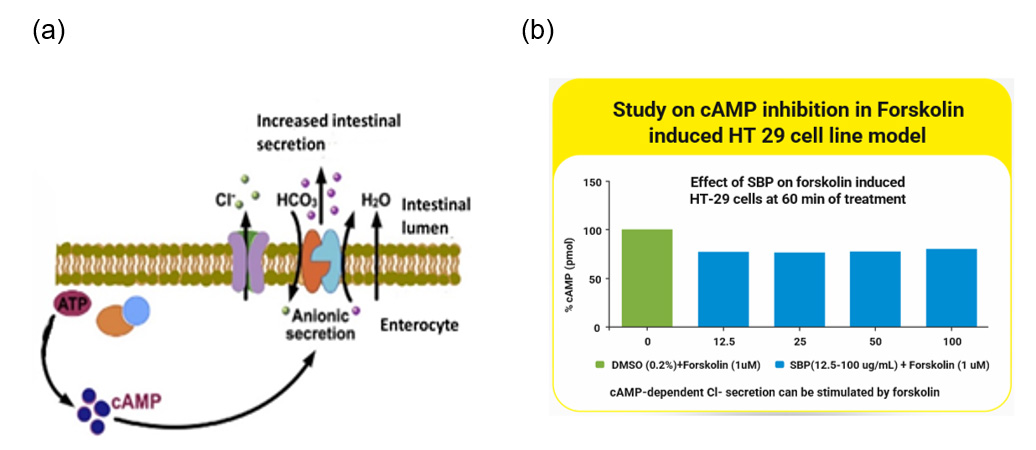
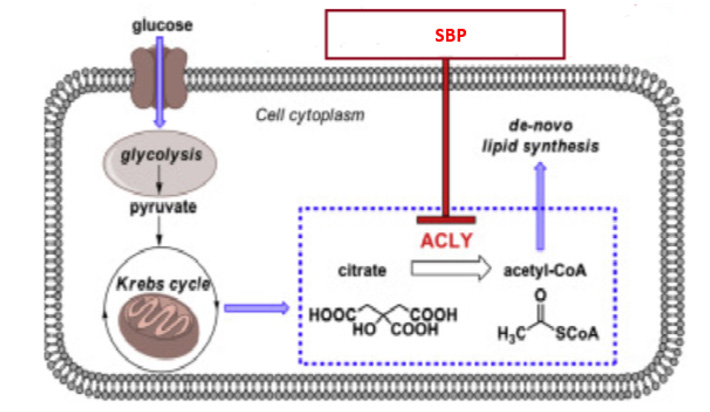
In the feasibility stage, the herbs that would address the specific issue are screened using in-vitro and in-vivo assays. If an SBP has been conceptualized, the potential herbs should undergo preliminary evaluation in cell lines and in experimental animals first to evaluate their biological efficacy. For instance, when formulating an SBP to maintain healthy microbiota and hence prevent loose litter in chickens, the astringent properties of the herb combination must be examined by in-vitro bioassays such as protein precipitation assay and half maximal effective concentration (EC50) values analysed followed by ex-vivo assays such as ileal loop assay to evaluate its effect on hypersecretion. Other relevant assays can be performed for the biomarkers of interest, such as inhibition of cAMP release response to Forskolin in HT-29 cell line (specific for enterocytes), as illustrated in Figure 1. Only those phytochemicals that pass this preliminary evaluation should be considered candidates for the developmental stage.
Next, in-silico assays can used to discover the best match among the possible herbs that passed the feasibility test. Phytochemicals showing synergistic effects should be tested in the biological systems for their efficiency. Specific animal disease models should be used to test the efficacy of the SBPs. The animal models may vary depending on the specific condition being addressed by the SBP. If the desired biological effect of the SBP is to be anti-diarrheal, the SBP should be tested in a well-established animal disease model such as magnesium chloride-induced, castor oil-induced, or non-saturated polysaccharides (NSPs) induced diarrhoea animal models, comparing the effect with a control group to test the efficacy of the SBP for fluid retention and zootechnical parameters as shown in Table 1.
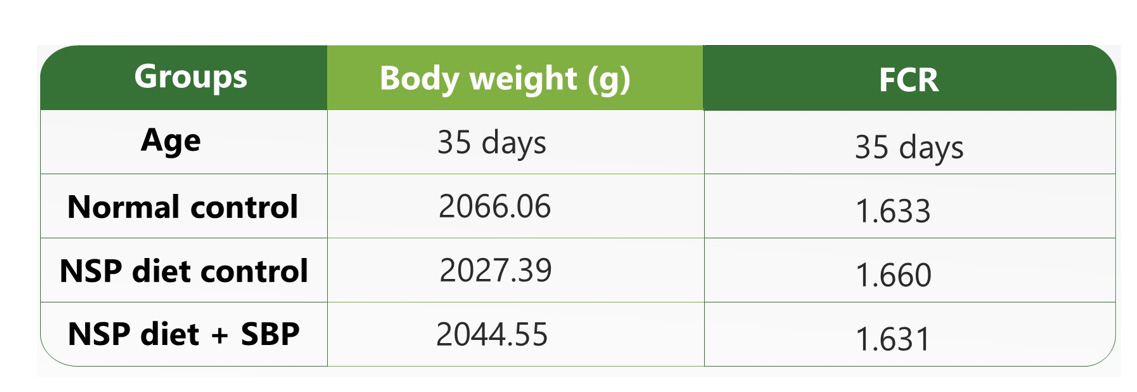
Table 1: Results of the zootechnical parameters of broiler birds with induced diarrhoea with NSPs and supplemented with SBP.
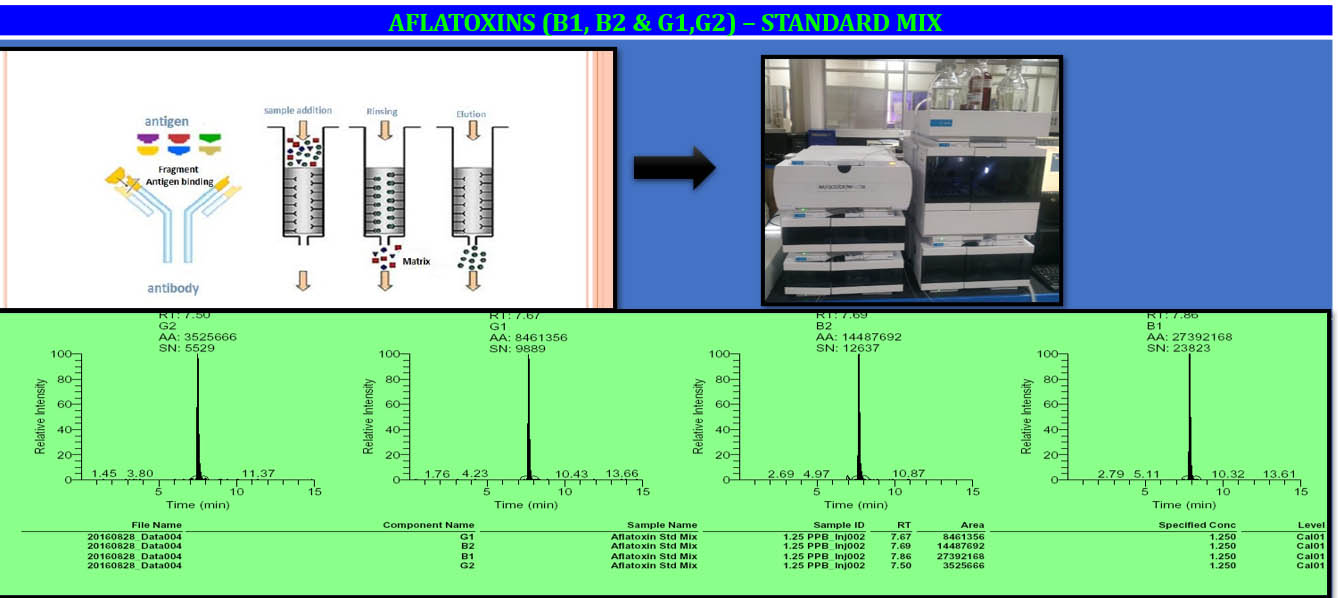
Following that, the herb formulation should be assessed for safety in smaller groups of target species in a controlled environment. The animals should be observed for any adverse reactions to the consumption of the SBP.
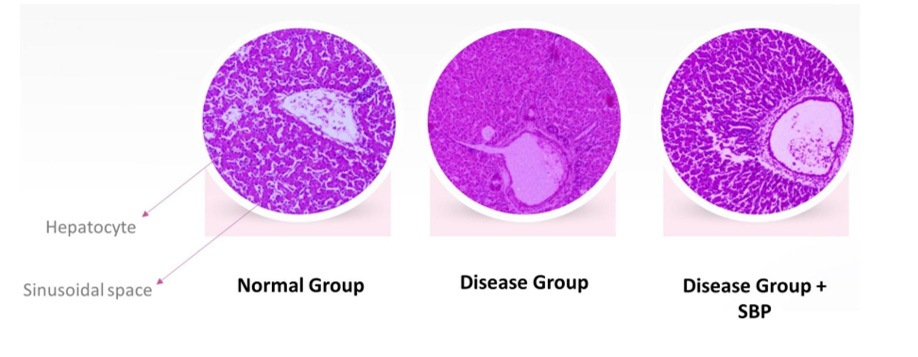
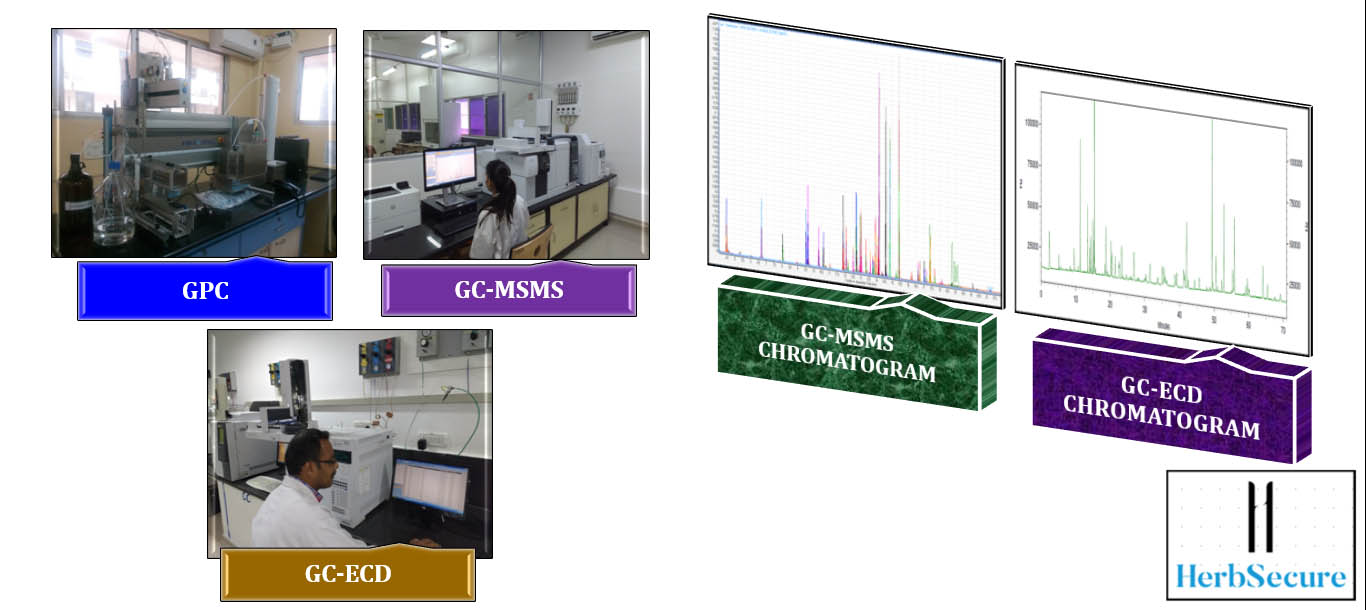
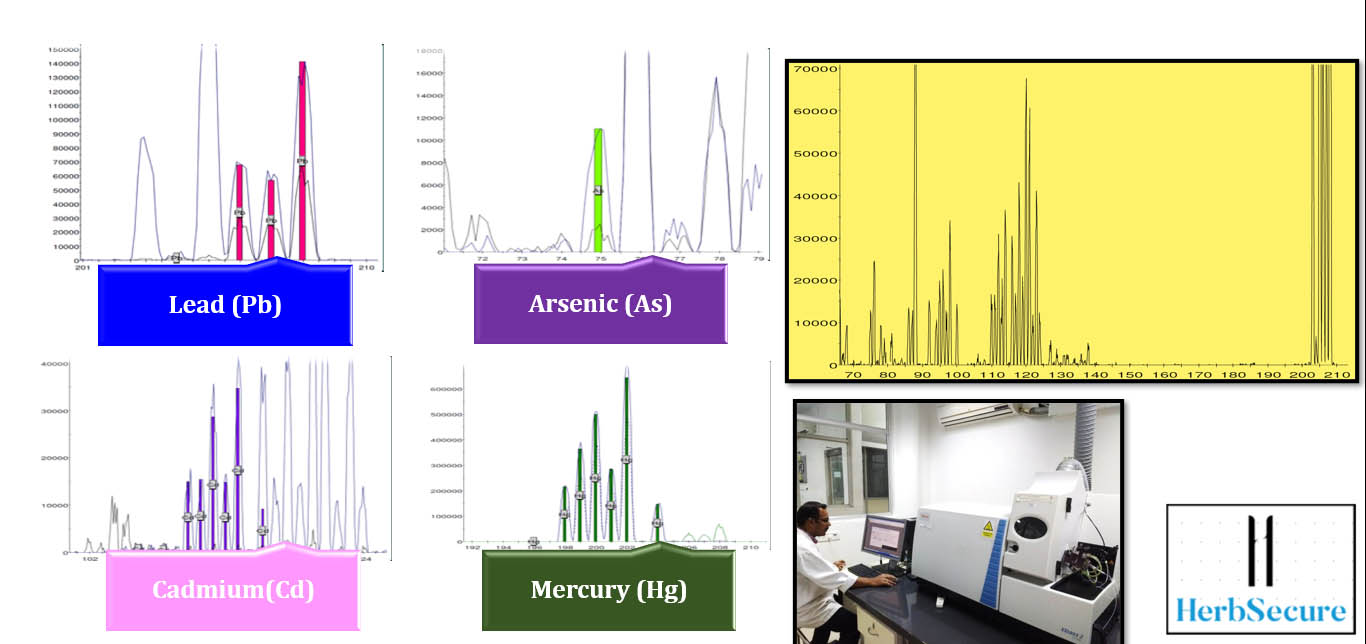
Only when the formulation passes this stage should it move to the scale-up stage, where larger populations of target animals are evaluated in the field conditions in different parts of the country and world. In this stage, the SBP is assessed for both its efficiency and its performance in response to the SBP, and zootechnical parameters such as body weight gain and feed conversion ratio (FCR). These parameters may vary depending on the SBP. The SBPs can also be evaluated for their effect on a single organ system through serological biomarkers, at the structural level through histological studies as shown in Figure 2, and at the molecular level using Omics studies as illustrated in Figure 3.
Omics studies
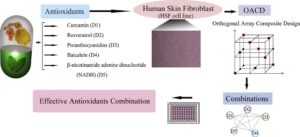
Omics studies are high throughput studies, comprising of proteomics, metabolomics, transcriptomics, etc. An in-depth understanding of pharmacodynamics, pharmacokinetics, and toxicological characterization of the active ingredients of a herbal product is gained through these research. The goal of omics studies is to identify and quantify large groups of biological molecules that are transformed in the structure, function, and dynamics of an organism.
Every material that is supplemented to an animal to achieve the intended result has a mechanism of action in the body, and it is critical to understand why and how it works. And, to answer the question, what genes, proteins, and metabolites in the body are affected by the substance in question? Omics studies are essential. This would provide scientific support and proof for the phenotypes displayed by the animals.
Considering natural compounds take a holistic approach to healing an animal, rather than focusing on a single molecule, a systems biological approach using OMICs studies is required to examine the herb’s effect on the complete system. Figure 3 illustrates the results of nutrigenomics in broiler breast muscle and liver after supplementation on Standardized Botanical Powders. Inferences on mechanism of action of the SBP can be drawn based on the gene alterations noticed in the treatment (SBP supplemented) group as compared to the disease (no supplementation) group.
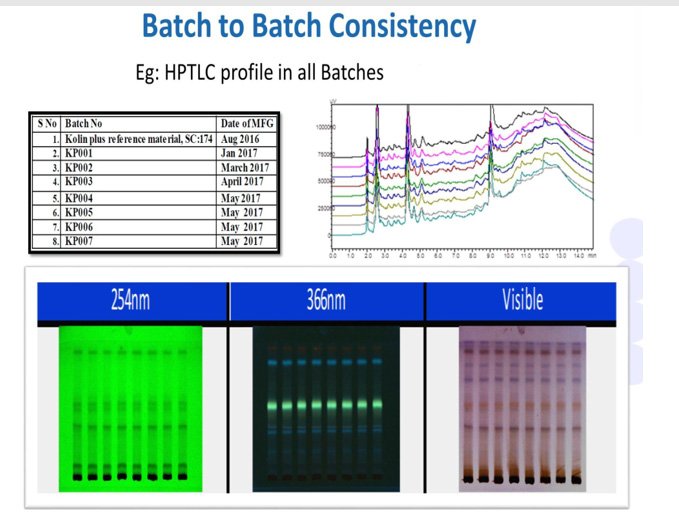
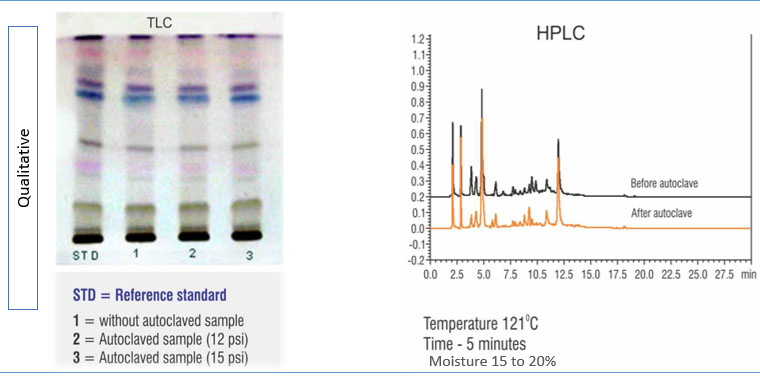
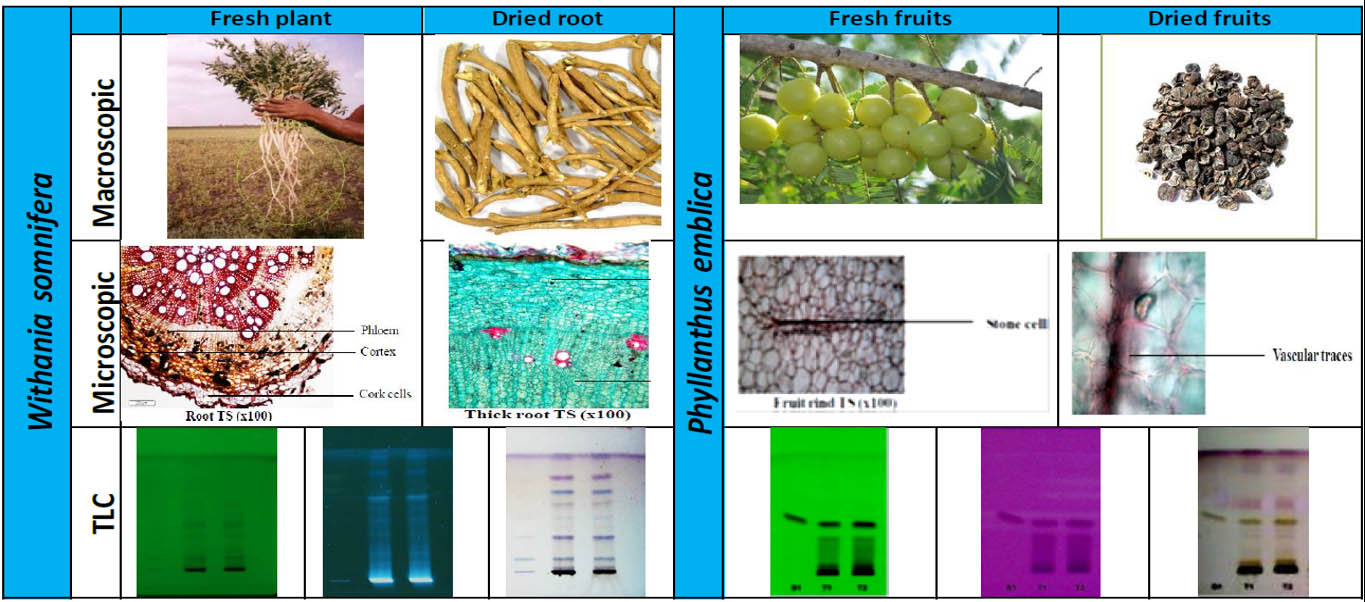
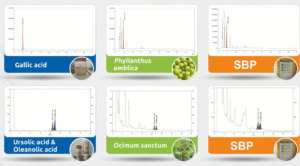
Batch-to-batch consistency must be evaluated to assess the efficiency of the standardization process. This can be verified with the help of chromatographic techniques where the products manufactured are compared against reference material of the product using an appropriate test method. As shown in Figure 4, high-performance thin-layer chromatography (HPTLC) is used to assess the phytochemical constituent pattern for batches manufactured during different periods.
To have a highly efficient and high-performing Standardized Botanical Powders, they must be scientifically assessed and evaluated thoroughly before and after their launch into the market.
To conclude, standardization is an essential process that ensures that active phytochemical concentrations are maintained with minimum fluctuation, ensuring efficient phyto-active function in the animal’s body. This helps monitor the product for consistency batch after batch so that it provides the expected results and the best performance in the animals.
Previous articles in this Series
PART 1: Standardized Botanical Powders : What? Why? How?
PART 2: Standardized Botanical Powder – To Be Or Not To Be
PART 3: Standardization Of Botanical Powders Starts At The Grassroots
PART 4: Check, Assessment & Revalidation Of SBPs





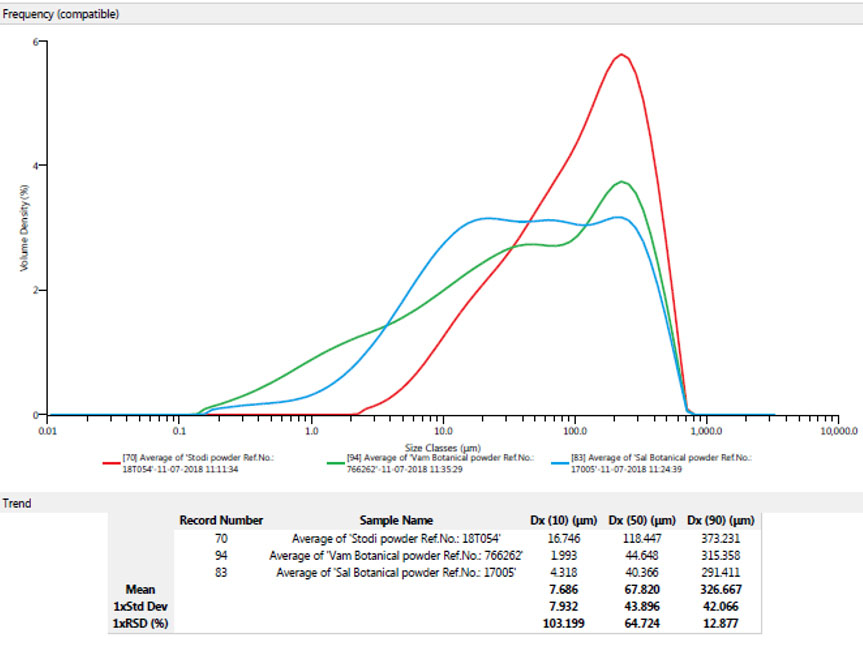

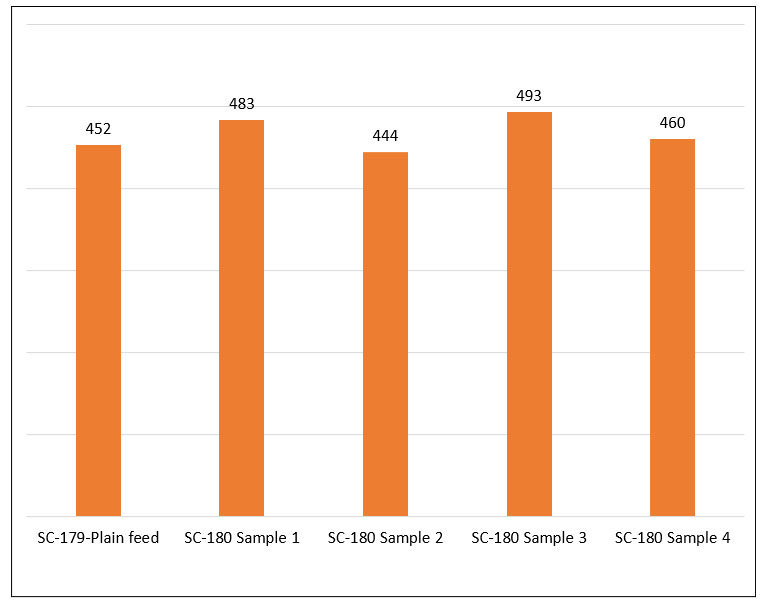







 health benefits associated with holistic living. The natural supplement industry is foreseeing a huge surge for evidence-based scientific herbal products. This growing market demands exceptionally high-quality and consistently efficient natural products.
health benefits associated with holistic living. The natural supplement industry is foreseeing a huge surge for evidence-based scientific herbal products. This growing market demands exceptionally high-quality and consistently efficient natural products.